Konference: 2015 20th Congress of the European Hematology Association - účast ČR
Kategorie: Mnohočetný myelom
Téma: Poster
Číslo abstraktu: P657
Autoři: MUDr. Viera Sandecká, Ph.D.; prof. MUDr. Roman Hájek, CSc.; prof. MUDr. Zdeněk Adam, CSc.; Prof.MUDr. Ivan Špička, PhD; Prof.MUDr. Vlastimil Ščudla, CSc.; MUDr. Evžen Gregora; MUDr. Jakub Radocha; Bc. Lucie Brožová; Jaroslav Jarkovský; Mgr. Lucie Říhová, PhD.; Mgr. Aneta Mikulášová; MUDr. David Starostka; MUDr. Lenka Walterová; MUDr. Dagmar Adamová; MUDr. Petr Kessler; MUDr. Martin Brejcha; MD Ivan Vonke; MUDr. Jarmila Obernauerová; MUDr. Kamila Valentová; prof. MUDr. Luděk Pour, Ph.D.; MUDr. Jiří Minařík, Ph.D. ; MUDr. Jan Straub; MUDr. Jaromír Gumulec; Doc. MUDr. Vladimír Maisnar, Ph.D.
Background
Two risk stratification models predict the progression from
smoldering multiple myeloma (SMM) to multiple myeloma (MM). i) the
Mayo Clinic model uses percentage of bone marrow plasma cells
(BMPCs) and serum monoclonal protein (M-protein) and free light
chain (FLC) ratio, ii) the PETHEMA model uses immunoparesis and the
percentage of abnormal PCs (aPCs) by flow cytometry.
Aims
The primary end point was to estimate the cumulative risk of MM
occurring during the follow-up of our cohort. The secondary end
points were: to validate known clinical models and to establish a
new risk model by the Czech Myeloma Group (CMG model) with better
prediction of ultra high-risk SMM group.
Methods
Data for this study were obtained from the Registry of Monoclonal
Gammopathies (RMG) acquired from hematologic centers of the Czech
Republic. In total, 361patients with SMM were enrolled in the RMG
study from May 2007 to June 2013. In total, 79.5% (287/361) of
patients were analyzed.
Results
287 SMM patients were followed with median 2.4 years. MM was
developed in 51.9% (149/287) of patients. The risk of progression
was 16%, 31.2%, 54.8% and 73.4% at 1, 2, 5 and 10 years after
diagnosis, respectively. The key predictors factors of progression
were as follows: serum (iFLC/uFLC) ratio >30 (HR 2.4 [1.4 -
4.1], p<0.001), BMPCs ≥15% (HR 2.1 [1.5-3.0]; p<0.001),
immunoparesis (HR 2.0 [1.3-2.9]; p<0.001), M - protein
concentration ≥2.3 g/dL (HR 2.00 [1.4-2.7]; p<0.001), beta2
microglobulin ≥2.0 mg/l (HR 1.8 [1.2-2.7]; p=0.001), and
thrombocyte count ≤250 x 109/l (HR 1.7 [1.1-2.4]; p=
0.005). Distribution of SMM patients according to risk groups based
on the Mayo Clinic model (Dispenzieri 2008) confirmed predictive
power of this model based on low and intermediate- risk group, but
did not confirm high-risk group. The high-risk group was
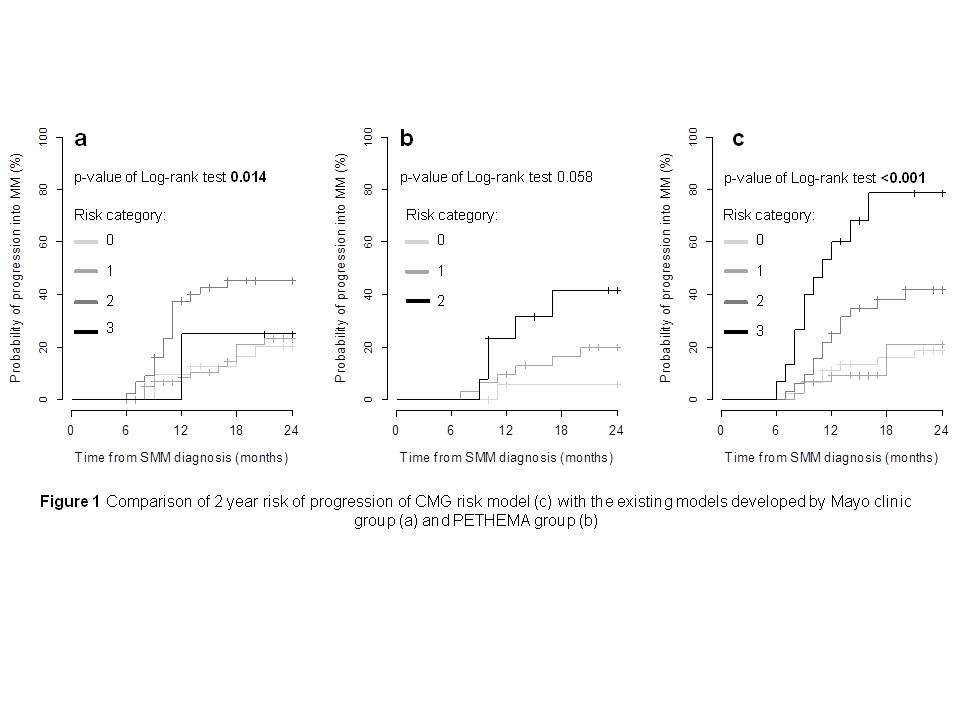
represented only by 4 SMM patients. At 2 years, no-risk group
had 20.2% risk of progression compared to 23.2%, 45.4% and 25% in
groups with 1, 2 or 3 risk factors, respectively (p=0.014) (Fig 1).
SMM group with 1, 2 and 3 risk factors in comparison to the
reference group had HR( 1.14 [0.54- 2.41]; p=0.732, HR 2.58 [1.25-
5.35]; p=0.011, HR 2.18 [0.47-10.03];p=0.317, n= 146),
retrospectively. Immunoparesis and ≥ 95% aPC was used to validate
the PETHEMA model (Perez-Persona 2007). The rates of progression at
2 years were 5.9%, 20% and 41.4% for groups with 0, 1 or 2 risk
factors, respectively (p=0.058) (Fig 1). SMM group with 1 and 2
risk factors in comparison to the reference group had HR (1.51
[0.40-5.69]; p= 0.546, HR 3.31 [0.78-14.06]; p= 0.104, n=62),
retrospectively. Based on the 3 parameters with independent
predictive value in the univariate analysis (immunoparesis, serum
M-protein quantity ≥2.3 g/dL and iFLC/uFLC >30) we proposed a
new CMG model. The risk of progression from SMM to MM at 2 years
was 18.5%, 20.9%, 41.9% and 78.7% if 0, 1, 2 or 3 risk
factors are present (p<0.001) with HR of 1.4 [0.7-2.9]; p=0.28,
2.5 [1.2-5.0]; p=0.008, 6.7 [3.0-15.2]; p<0.001, n=139),
respectively (Fig 1).
Summary
We confirmed the validity of previously considered clinical models
by the Mayo Clinic group (except high-risk group) and the PETHEMA
group. New CMG model for the risk of progression from SMM to MM was
established. Better identification of ultra high-risk group with
prediction of 79% risk of progression to MM within two years based
on easily accessible clinical parameters is advantage.
Acknowledgments: NT13492-4, NT14575-3 and by EU FP7/2007-2013; grant 278570, IGA MZ CR NT14393.
Datum přednesení příspěvku: 13. 6. 2015