Konference: 2015 57th ASH Annual Meeting - účast ČR
Kategorie: Maligní lymfomy a leukémie
Téma: 615. Acute Myeloid Leukemia: Commercially available Therapy, excluding Transplantation: Poster I
Číslo abstraktu: 1336
Autoři: MD Agnieszka Wierzbowska, PhD; Ewa Wawrzyniak, Ph.D.; Prof. Dr. Agnieszka Pluta; MD Tadeusz Robak, PhD.; MD Grzegorz Mazur, PhD; Anna Dmoszynska; prof. MUDr. Jaroslav Čermák, CSc.; M.D. Albert Oriol; MD Farhad Ravandi Ravandi; M.D. Hagop M. Kantarjian
Objective: To determine the effects of treatment with DEC vs low-dose cytarabine or best supportive care (BSC) as a treatment of choice (TC) on OS, progression free survival (PFS) and response rate (CR/CRp) in the subset of pts with AML MK+, who were treated in the randomized phase III DACO-16 study. A second objective is to compare the OS, PFS and CR/CRp of MK+ vs other unfavorable karyotype cases.
Methods: Eligible pts were ≥65 years old with newly diagnosed, de novo or secondary AML with ≥20% bone marrow blasts, ECOG performance status 0-2, WBC count ≤40x109/L. According to study protocol pts were randomly assigned 1:1 to receive DEC 20 mg/m2 per day as a 1-hour i.v. infusion for five days every 4 weeks or TC (BSC or cytarabine 20 mg/m2 s.c. for 10 days every 4 weeks). This analysis included pts identified from the clinical database whose locally obtained cytogenetics data indicated they had MK+. Pts with poor-risk cytogenetics (Southwest Oncology Group classification) but MK- (poor-risk MK-) were used as the comparison group. Median OS (Kaplan-Meier method) and PFS were compared between decitabine vs TC treatment arms for pts MK+ and poor-risk MK-. Remission was assessed using modified IWG AML criteria (Cheson, Blood, 2003).
Results: Of 480 pts in DACO-16 study, 64 (13.3%) fulfilled the criteria for MK+ and additional 99 (20.6%) had poor-risk MK- cytogenetics. In MK+ group 33 pts received DEC and 31 pts were assigned to TC. In poor-risk MK- group DEC and TC was administered to 49 and 50 pts respectively. Baseline characteristics of treatment subgroups were comparable.
Outcome of MK+ and poor-risk MK- pts according to treatment arm
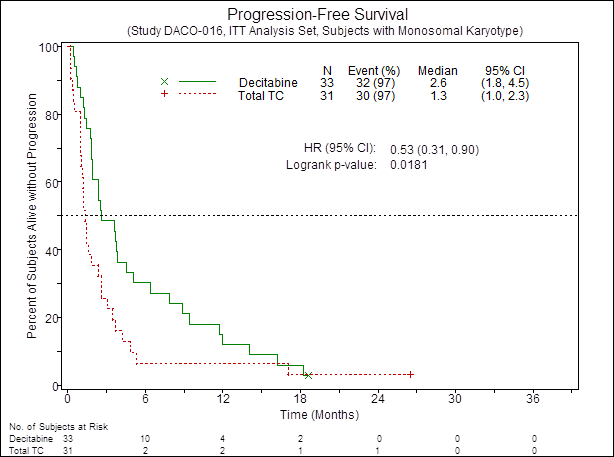
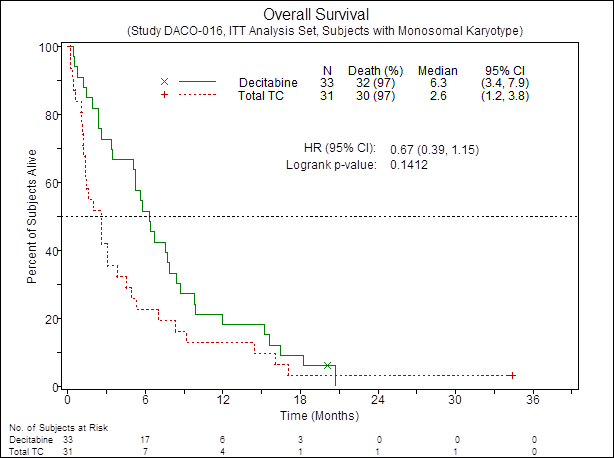
The CR/CRp rate in MK+ pts was higher in the DEC arm compared to TC (21% vs 3%; OR=8.1; 95% CI, 0.93 to 70.04; P = 0.054). The median PFS was also improved in the DEC arm vs TC (2.6 vs 1.3 mos, HR=0.53; 95% CI, 0.31 to 0.9; P = 0.018) Fig.1. There was a trend towards longer OS by ~4 mos in the DEC arm vs TC (2.6 vs 6.3 mos; HR=0.67; 95% CI, 0.39 to 1.15; p=0.14) Fig.2. In contrast, no difference in CR/CRp rate between DEC vs TC was observed in poor-risk MK- pts (respectively 8% vs 10%; OR=0.8; 95% CI, 0.2 to 3.17; P = 1.0). Similarly, no improvement in PFS as well as OS was observed in poor-risk MK- cases treated with DEC compared to TC.
Overall survival in decitabine and TC treatment arms according to cytogenetics
In AML patients receiving low-dose cytarabine or BSC, patients with MK+ appeared to have a poorer outcome than pts with poor-risk MK-. The median OS was 2.6 vs 4.7 mos (HR=1.52; 95% CI, 0.91 to 2.54; p=0.11) in MK+ and poor-risk MK- pts, respectively. In contrast, in the DEC arm MK+ pts did not do worse than MK- cases with poor-risk cytogenetics. The median OS was similar: 6.3 vs 5.0 mos for MK+ and poor-risk MK- (HR=0.98; 95% CI, 0.6 to 1.58; p=0.92), respectively.
Conclusions: In older patients with AML-MK+, decitabine improved CR/CRp rate and PFS compared with standard therapies. A survival analysis suggesting a benefit of about 4 months in median OS for decitabine in MK+AML needs confirmation in further studies with larger sample size. These data might suggest decitabine overcomes the extremely poor prognosis associated with MK and may be a beneficial initial treatment as an alternative to low-dose cytarabine or best supportive care.
Figure 1. PFS of patients with AML MK+according to treatment
Figure 2. OS of patients with AML MK+ according to treatment
Disclosures: Off Label Use: Decitabine in elderly AML patients. Robak: Eisai Inc: Research Funding . Oriol:Celgene, Janssen, Amgen: Consultancy , Speakers Bureau .
Datum přednesení příspěvku: 5. 12. 2015