Konference: 2015 57th ASH Annual Meeting - účast ČR
Kategorie: Maligní lymfomy a leukémie
Téma: 731. Clinical Autologous Transplantation: Results
Číslo abstraktu: 517
Autoři: M.D. Eric Van den Neste, Ph.D.; MD Norbert Schmitz; M.D. Nicolas Mounier, Ph.D.; Devinder S Gill, MB ChB, MRCP (UK), FRCPath (Lond); Prof. MUDr. Marek Trněný, CSc.; MD Noel-Jean Milpied, Ph.D.; Prof. M.D. John Radford; Nicolas Ketterer; MD Ofer Shpilberg, MPH; M.D. Ulrich Dührsen (Duehrsen); Prof. Dr. David Ma; M.D. Josette Briere; M.D. Catherine Thieblemont, Ph.D.; MD Gilles Andre Salles, PhD; M.D. Craig H Moskowitz; M.D. Bertram Glass, PhD; MD Christian Gisselbrecht
Results:
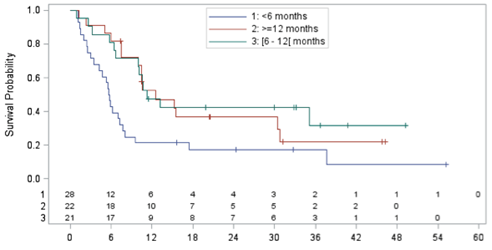
Median time between ASCT scheduled in CORAL and relapse was 7.1 months (range 3.1-61.9) with 32.9% relapsing > 12 months. Median age was 56.1y (range 20.9-67.7), M/F ratio 51/24, sIPI 0-2 in 71.6%, >2 in 28.4%. 49.3% were in the rituximab and 45.3% in the observation arm of the CORAL. All patients had previously received rituximab. Third-line therapy consisted of ICE-type (17.3%), DHAP-type (24%), gemcitabine-containing (28%), CHOP-like (13.3%), and miscellaneous regimens (17.3%). Overall response rate to third-line chemotherapy was 44%, with 32% complete response (CR)/CR unconfirmed (CRu), and 12% PR. Among the 75 patients, 16 (21.6%) could eventually be transplanted 3 ASCT and 13 allogeneic SCT with conditioning regimens includingfludarabine. Median OS, calculated from time of relapse until death, was 10.0 months (95% CI 6.6-12.6; min: 0.9-max: 55.2 months; median follow-up: 32.8 months) with an estimated 1-y OS of 39.1%. Median OS was statistically different (p=.0007) according to sIPI at CORAL failure: sIPI 0-2: 12.6 months (1-y OS 51.3%), sIPI > 2: 5.3 months (1-y OS 21.6%, HR 2.805). Median OS in patients achieving CR/CRu, PR, or no response after third-line regimen was 37.7 m (1-y OS 90.5%, p<.0001, HR 0.132), 10.0 m (1-y OS 44.4%, p=.03, HR 0.375), and 6.3 months (1-y OS 13.4%), respectively. Median OS of patients who could eventually be transplanted was 17.4 months (1-y OS 68.2%), as compared to a median OS of 8.0 months in those who were not transplanted (1-y OS 31.2%) with a HR of 0.575 (p=.11). Median OS was particularly dismal among patients who relapsed < 6 months after CORAL-scheduled ASCT (5.7 months, n=28), as compared to those relapsing either > 6 and < 12 months (11.3 months, n=21) or > 12 months after ASCT (12.6 months, p=0.01, fig. 1)). In multivariate Cox analysis (with the following variables entered: age, sex, sIPI, response to third line and time between CORAL ASCT and relapse),sIPI >2 (HR 2.464, p=0.01), achievement of CR (HR 0.1, p<.0001) or PR (HR 0.242, p=0.02), and post-ASCT remission lasting < 6 months (HR 2.270, p=0.05) independently predicted for OS.
Conclusions: Overall, the outcome of DLBCL patients relapsing after second-line R-DHAP/R-ICE followed by ASCT is poor. However, prognostic factors predicting better outcome in this group are late (> 6 months) relapse, lower sIPI and achieving at least PR after third-line salvage followed by transplantation. In the transplanted patients (allo SCT or second ASCT) a 2-year OS of 50% was observed. Thus, new salvage regimens can be a bridge to transplantation in patients with late relapse and/or low sIPI,. New drugs improving salvage efficacy are urgently needed, especially for patients relapsing < 6 months following ASCT.
Figure 1: OS (months) in DLBCL patients relapsing after CORAL-scheduled ASCT according to interval between ASCT and relapse (<6 months, 6-12 months, > 12 months)
Disclosures: Schmitz: Roche, Takeda, Gillead, Riemser und ctilifesciences: Other: Advisory board , Speakers Bureau . Gill: Sanofi Aventis: Research Funding ; Roche: Honoraria ; AbbVie: Honoraria ; Roche: Research Funding . Milpied: Celgene: Honoraria , Research Funding . Briere: St. Louis Hospital, Paris, France: Employment . Thieblemont: St. Louis Hospital, Paris, France: Employment . Salles: Celgene Corporation; Roche and Gilead Sciences: Research Funding ; Celgene Corporation; Roche: Speakers Bureau ; Calistoga Pharmaceuticals, Inc.; Celgene Corporation; Genentech, Inc.; Janssen Pharmaceutica Products, L.P.; Roche: Consultancy . Gisselbrecht:roche: Research Funding , Speakers Bureau .
Datum přednesení příspěvku: 7. 12. 2015